Unveiled in August 2020, the Just5 jacket(1) provides protection against the SARS-Cov-2 virus, the pathogen responsible for the covid-19 pandemic, and is one of the latest chapters in the fast-moving story of a commercial textile treatment (Viroblock NPJ03) that was little more than an idea at the end of 2019. The treatment’s speedy commercialisation is the work of HeiQ of Schlieren, Switzerland(2). It is also a story that echoes many of the profound changes we have all experienced in the last few extraordinary months.
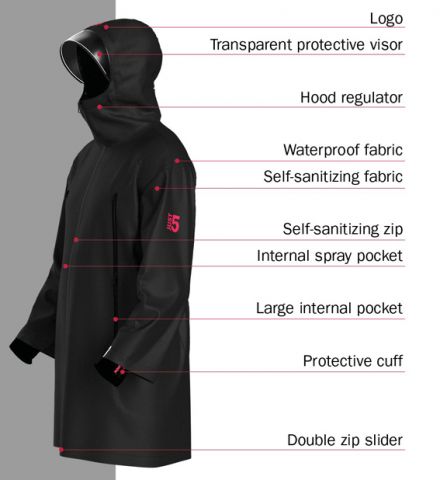 The Just5 jacket serves as a showcase for the technologies being developed by HeiQ and its partners using Viroblock NPJ03.
The Just5 jacket serves as a showcase for the technologies being developed by HeiQ and its partners using Viroblock NPJ03.HeiQ was founded in 2005 by two former employees of the Zurich-based Swiss Federal Institute of Technology (commonly known as ETH): Carlo Centonze is now Chief Executive Officer (CEO); Murray Height is Chief Scientific Officer (CSO). During a hike in the Swiss mountains with their life partners, the two co-founders decided to find a way to treat textiles to suppress the odours generated by their physical activity.
They wanted to use science to help manufacturers make clothes that stay fresh for several days and succeeded by applying a silver-based treatment to polyester (PES) fabrics. The positive electrical charge of the silver ions attracts and binds them to the oppositely charged membranes of the bacteria responsible for the odours, which proliferate in the warm, moist sweat generated by exercise. The bound silver causes structural damage to the membranes, leading to the death of the microbes.
The company name is in part derived from that “hike”, but also represents “high-quality” materials and high intelligence quotient (“high IQ”), an allusion to intelligent materials and technologies. Centonze and Height describe HeiQ as a three-in-one company, which aims to develop treatments that improve the functionality of textiles based on: scientific research (currently, more than 40 PhD students are engaged in work relating to HeiQ projects); the manufacture of speciality materials; consumer branding of products. Centonze also likens the company to the Swiss Army knife (a multi-tool pocketknife manufactured by Victorinox of Ibach, Switzerland), providing customers with all the tools and services they need in one source.
By 2019, HeiQ’s portfolio included about 200 products distributed among several brands:
- HeiQ Pure—a silver-based finish to combat odours;
- HeiQ Fresh—a silver-free treatment to help control odours and volatile organic compounds (VOCs);
- HeiQ Smart Temp—for thermoregulation;
- HeiQ Eco Dry—perfluorocarbon (PFC)-free repellents to water and water-based stains;
- HeiQ XReflex—a radiant barrier to enhance a material’s thermal insulation;
- HeiQ Clean Tech—an additive to reduce waste and costs during the dyeing of PES;
- HeiQ Real Silk—a treatment to give textiles the soft, dry, cool and comfortable feel of silk.
It had also grown in geographical reach, employing more than 90 staff in 12 countries on five continents. The company has production facilities in: Bad Zurzach, Switzerland; Geelong, Australia; Concord, North Carolina, and Calhoun, Georgia, USA; as well as additional offices in: Maia, Portugal; Shanghai, China; Taoyuan City, Taiwan. In addition, it has official distributors in the USA, Singapore, South Korea, Sri Lanka, Turkey, Guatemala, Thailand, Indonesia, Bangladesh, India, Pakistan, Mexico, the Republic of Mauritius and Japan.
Customers include major global retailers and brands in: sports and outdoor wear (including Speedo, Burton and Patagonia); intimate apparel and hosiery (such as Triumph, Sloggi and Hanes); fashion (for instance, Champion, Gap and Marks & Spencer); domestic textiles (including Bekaert Deslee, American Textile Co and Sealy); footwear (for example, New Balance); workwear (Dickies, Duluth Trading and Carhartt).
Quick turnaround
By December 2019, however, the company had recognised the growing threat of a global pandemic and switched its attention to finding a way to help to prevent the spread of the SARS-Cov-2 virus, which it is believed can persist on surfaces such as those of textiles for a few days(3). As a result, even face masks worn to help prevent the airborne spread of the disease can be a cause for concern, because of the threat of transferring viruses accumulated on their outer surfaces to vulnerable points on the wearer’s skin.
Healthcare professionals, for instance, are trained in the use of masks, such as the need to wash their hands after doffing them, but while working under extreme pressure can forget to follow this advice, and the threat to untrained users can be even greater. In part, such concerns prompted the World Health Organization (WHO) of Geneva, Switzerland, to recommend in the early days of the pandemic against the routine use of face masks by healthy members of the public(4).
Consequently, HeiQ began to produce a treatment that would rapidly kill the virus on contact with fabric surfaces. Centonze proudly recounts how it did so in a very short space of time: from the production of the first batch to the onset of global distribution took just six weeks, a process that would typically take one-and-a-half to two years.
Combined in a patent-pending development, the breakthrough exploits two of the company’s pre-existing technologies:
- its registered silver-based antiviral and antibacterial treatment;
- a technology based on the use of fatty vesicles or liposomes (originally introduced in 2011).
As above, the silver ions attract the viruses and bind to the sulfur groups in their structures, but in this case, the breakdown of the pathogens is assisted by the presence of the liposomes. While the fatty membranes of the liposomes resemble those of the viruses, they differ significantly by enclosing only empty spaces. This establishes a concentration gradient between the bare interiors of the liposome particles and any chemicals within adjacent viruses, which results in cholesterol within the latter being drawn out. The depleted virus is brittle, and the accelerated destruction of its membrane leads to the oxidisation of its exposed ribonucleic acid (RNA).
 Two laboratory tests were developed to compare the residual infectivity of the SARS-Cov-2 virus after remaining in contact with treated and untreated (inoculum) fabrics for 30 minutes. *TCID50 (tissue culture infected dose50) is a standard measure of the number of infected cells in a given volume.
Two laboratory tests were developed to compare the residual infectivity of the SARS-Cov-2 virus after remaining in contact with treated and untreated (inoculum) fabrics for 30 minutes. *TCID50 (tissue culture infected dose50) is a standard measure of the number of infected cells in a given volume.The synergistic effect is unique, according to Centonze, and rapidly results in destruction of viruses (as well as bacteria and yeasts), helping to prevent contamination of and transmission via the treated surface. One early test of the efficacy was conducted according to the ISO 20743: 2013 Textiles—Determination of antibacterial activity of textile products(5) method published by the International Standards Organization (ISO) of Geneva, Switzerland, but modified to use the Murine respirovirus, (formerly known as the Sendai virus). This showed that the residual infectivity was insignificant after only two-to-five minutes of contact between the treated nonwoven fabric and a viral load.
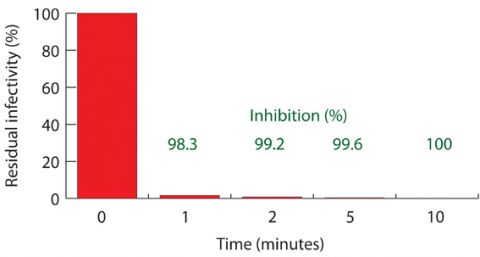 The residual infectivity of a Sendai virus in contact with a nonwoven fabric treated with Viroblock was measured over time.
The residual infectivity of a Sendai virus in contact with a nonwoven fabric treated with Viroblock was measured over time.Further testing, reported by HeiQ in March 2020(6), showed that the treatment is highly effective against the 229E strain of the human coronavirus, which has a membrane structure like that of SARS-Cov-2. These tests showed that within minutes of contact, treated face masks had a 99.99% reduced level of infectivity compared with untreated ones.
By this time, momentum was building and several companies were working to incorporate the treatment into their products, including:
- Suzhou Bolisi of Zhangjiagang, China—a manufacturer of protective masks;
- Kayser-Roth of Greensboro, North Carolina, USA—traditionally a producer of legwear, which had begun making hand protectors (called Ghluv) in response to the pandemic;
- Lufeng of Zibo, China—a manufacturer of apparel fabrics.
Demand soon outstripped HeiQ’s capacity to supply. Since the official launch on 16 March 2020, its four plants had been running at full capacity making 145 t a day. To make more available, therefore, the company issued free licences to three of its competitors(7):
- CHT Group of Tübingen, Germany;
- Jintex Group from Taipei, Taiwan;
- Piedmont Chemical Industries based in High Point, North Carolina, USA.
To safeguard the product’s reputation in a turbulent climate, when the claims of many manufacturers were being openly challenged and accusations of impropriety were even being made by world leaders (see also, Industry responds urgently and rapidly to the global pandemic), the company stipulated the licensees must have their treated fabrics tested by accredited third-party institutes to establish the efficacy and quality as per HeiQ’s standards. In addition, use of its HeiQ Viroblock trademark with respect to treated articles required the company’s authorisation.
 Left to right: David Juang, Chairman of Jintex; Ralf Kattanek Group Vice President Textiles of CHT; Carlo Centonze, Chief Executive Officer (CEO) of HeiQ. Image courtesy of HeiQ.
Left to right: David Juang, Chairman of Jintex; Ralf Kattanek Group Vice President Textiles of CHT; Carlo Centonze, Chief Executive Officer (CEO) of HeiQ. Image courtesy of HeiQ.Currently, HeiQ has the capacity to make 250 t a day, but Centonze says that the three licences will remain in force as long as the terms are respected.
The company’s caution was justified when, in June 2020, HeiQ reported some products on the market purporting to be treated with Viroblock were fake(8). In response, it threatened to instigate legal proceedings, urged buyers to be wary and offered to help prospective customers of products claiming to be protected by Viroblock to check their authenticity. To date, the company has issued at least a dozen letters demanding companies cease making their false claims, which it says have all been heeded without the need for further action, but HeiQ continues to monitor for such misrepresentations and to protect its intellectual property.
Testing achievement
Just a few days before, HeiQ had announced an important milestone had been achieved: tests at the world-leading Peter Doherty Institute for Infection and Immunity in Melbourne, Australia, had confirmed the treatment is effective against the SARS-Cov-2 virus(9). Centonze believes his company was probably the first to test and to establish such an effect by a treated textile against the virus responsible for covid-19 and was able to do so thanks to its strong partnership with the Institute.
Based on the ISO 18184 Textiles—Determination of antiviral activity of textile products standard, adapted for the SARS-Cov-2 virus, the partners were able to develop two different test protocols, which demonstrated the infectivity was reduced by 99.99% after 30 minutes of contact with a treated woven PES fabric, compared with the control (an untreated woven PES fabric).
To test the effect of the treatment on face masks, HeiQ chose a high-quality product manufactured in China, one satisfying the FFP2-level requirements of the European standard EN 149: Respiratory protective devices—Filtering half masks to protect against particles—Requirements, testing, marking. Tests on several masks were conducted according to the ASTM F2101 Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus(10). These test methods, published by the ASTM of West Conshohocken, Pennsylvania, USA, were adapted for use with viruses and are lengthy and expensive to conduct, but the most meaningful in this context, HeiQ says.
Conducted according to the principles of good laboratory practice(11) using a dozen different microorganisms, the data showed a reduction in the transmission of viable particles through the masks (treated against untreated) of log 2.5–3.5. For an exposure of 100 000 droplets, roughly equivalent to the amount anticipated from a single cough(12), the results suggest untreated masks would permit more than 23 viable droplets to pass through them, compared with fewer than one on average for treated masks.
 A cough releases about 100 000 droplets into the air and to assess the protection offered by face masks when exposed to them all, a method for judging how many viable droplets (those capable of causing infection) pass through was devised. Face masks treated with Viroblock are more than 20 times as effective as untreated (control) ones, HeiQ concludes. The studies were conducted by Microbac Laboratories in Sterling, Virginia, USA, and fuller details (Study ID 798-110) can be found on HeiQ's website: https://heiq.com/heiq-viroblock-npj03-test-reports/.
A cough releases about 100 000 droplets into the air and to assess the protection offered by face masks when exposed to them all, a method for judging how many viable droplets (those capable of causing infection) pass through was devised. Face masks treated with Viroblock are more than 20 times as effective as untreated (control) ones, HeiQ concludes. The studies were conducted by Microbac Laboratories in Sterling, Virginia, USA, and fuller details (Study ID 798-110) can be found on HeiQ's website: https://heiq.com/heiq-viroblock-npj03-test-reports/.While the untreated masks clearly offer effective filtration, Centonze notes that just a few viral particles are sufficient for a person to become infected and that the level of protection of the treated masks assessed in this manner is more than 20 times higher.
The Viroblock treatment can be applied to all types of fibres and fabric constructions, and crucially is durable to washing. HeiQ claims the treatment remains effective for 30 domestic washes at up to 60°C (140°F). Consequently, it is suitable for applications other than face masks, including:
- medical nonwovens (such as surgical gowns, scrubs, drapes and curtains);
- apparel;
- domestic textiles;
- textiles used on public transportation;
- air filters.
The initial focus was to supply manufacturers of face masks for healthcare workers, but increasing consumer demand meant that by the middle of June 2020, more than 1000 companies(13) were looking to use Viroblock to make a variety of products. These included major manufacturers of fashionwear (Burberry), intimate apparel (Hanes), shirting (Albini) and mattresses (Serta Simmons(14)).
Meanwhile, HeiQ’s China office had set-up a manufacturing capacity for treated face masks, beginning shipments in March 2020. By the middle of June, it had shipped 300 million units for the public and another four million high-quality masks suitable for healthcare workers.
Celine Huang, CEO in China, explained that, although this is not the company’s core competence, it felt a duty to help address the shortages of personal protective equipment (PPE) caused by the severe disruption to global supply chains. Working with Chinese partners, HeiQ succeeded in finding a way to use the padding method to apply the treatment to a variety of fabrics, even nonwovens where the lack of warp and weft fibres makes it difficult to maintain a uniform tension in the material during processing. In this way, the company can apply 5–20% by weight of the treatment to a fabric.
Testing of the treated fabrics, according to the standard ISO 18184, proved that they reduced the viral activity by 99.99% in a few minutes. Treated and untreated FFP2 face masks were also compared with an aerosol challenge test featuring a variety of viruses. These studies showed that, depending on the virus, the treatment reduced the infectivity by 30–200 times.
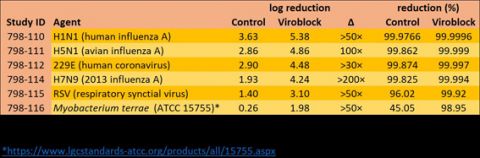 The protection offered by high-quality face masks against aerosols carrying one of several viruses or a bacterium was compared for treated and untreated (control) products. These studies were conducted by Microbac Laboratories in Sterling, Virginia, USA, and fuller details can be found on HeiQ's website: https://heiq.com/heiq-viroblock-npj03-test-reports/.
The protection offered by high-quality face masks against aerosols carrying one of several viruses or a bacterium was compared for treated and untreated (control) products. These studies were conducted by Microbac Laboratories in Sterling, Virginia, USA, and fuller details can be found on HeiQ's website: https://heiq.com/heiq-viroblock-npj03-test-reports/. 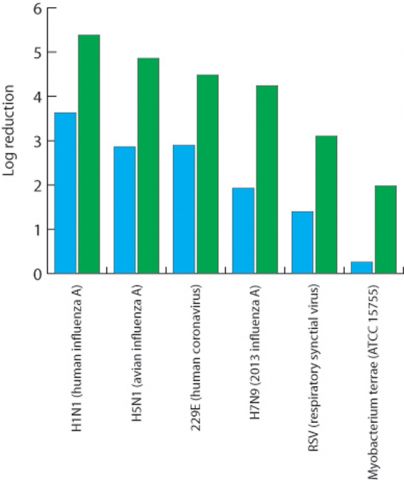
In China, the company makes and supplies disposable (maximum wearing time eight hours) masks in a variety of styles. In Switzerland, meanwhile, it makes two versions of a washable (up to 30 times at 40°C) three-layer mask. In this case, the outer and inner layers are both PES knitted fabrics and the double-layer filter in the middle is a water-repellent meltblown treated with Viroblock and conforming to the requirements of Europe’s toughest such standard EN 14683:2019 IIR Medical face masks—Requirements and test methods. The outer layer is also treated with Viroblock, while the inner face is a soft, untreated fabric to make the mask comfortable to the wearer’s skin.
For sale in the USA, one of the disposable masks (designated HVB-FFP2-01) has already been approved by the Food and Drug Administration (FDA) of Silver Spring, Maryland, under its Emergency Use Authorization regulations(15), making HeiQ the first authorised Swiss supplier of such products. Currently, the others are pending approval.
More chapters to follow
One of the latest, but already not the last(16), parts of this story is the development of the Just5 jacket, a result of HeiQ’s collaboration with four specialist partners:
- 2A-NyGuard SpA of Borgaro, Italy, which has helped to develop the self-sanitising zips (NyShield);
- Coats of London, UK, which has incorporated Viroblock into its sewing threads to make every stitch in the jacket antiviral and antibacterial;
- Sitip of Cene, Italy, which has developed a treated warp-knitted fabric for use on the jacket’s extendable cuffs;
- Vagotex SpA of Colognola ai Coll, Italy, which has combined the treatment with its waterproof, windproof, but breathable laminate fabrics (Windtex).
All the elements of the jacket render viruses and bacteria landing on them ineffective within five minutes of contact. In addition, HeiQ has signed an agreement granting Coats, a specialist in industrial yarns and threads, exclusive global rights to use Viroblock on its products(17).
According to Centonze, the consensus of those developing vaccines for covid-19 is that even if they are successful soon it will be 18–24 months from now before this approach can become effective in managing the current pandemic. Further, following the outbreak in China in late 2002 of the related disease SARS(18), the habits of many in the most affected region (southeast Asia) changed permanently and even after it was suppressed, they continued to use PPE, particularly face masks. Virologists, meanwhile, expect further epidemics and pandemics will occur. All of which suggests that, as rich as it is already, the story of Viroblock has many more chapters to be written.
